
3.2 Soil Acidity and Liming
See also 2.3 Soil pH
What is soil acidity?
Soil acidity occurs when there is a build up of acid in
the soil. The production of acid in the soils is a natural process and
many soils in the high rainfall areas of SA are inherently acidic.
Acidification is a slow process but it is accelerated by agriculture.
As soils become more acidic, plants intolerant of acidic
conditions do not thrive and productivity declines.
How fast does soil acidify?
The rate at which a soil acidifies depends
on:-
-
Soil type
Light sandy soils with little clay or
organic matter have lower buffering capacity and therefore acidity
develops more quickly than on heavier soils.
-
Rainfall
Higher rainfall increases leaching of
nutrients which in turn increases acidification.
-
Land use
Higher production increases the rate of
acidification. Shallow rooted plant systems also increase
acidification compared with deep-rooted plants.
Figure 1:
Examples of acidification rates for
different farming systems
|
Farming system
|
Acidification rate*
|
|
Extensive grazing
|
10-25
|
|
Improved pasture
|
50
|
|
Cropping
|
75-100
|
|
Cropping with high N input
|
400
|
|
Horticulture with high N input
|
up to 500
|
|
Typical hay paddock
|
300
|
|
Lucerne hay
|
500-600
|
*kg lime/ha/year required to neutralise
acidity
What causes soil acidity?
The natural rate of acidification is
accelerated by agricultural practices. These include: Use of nitrogen
fertilisers The impact of nitrogen fertilisers on acidification depends on
the type of fertiliser and what happens to the nitrogen (see Table 2).
Figure 2:
Lime required to counteract
acidity caused by fertilisers in acid soils.
|
Fertiliser
|
Acidification
(kg lime/kg of N (or S) fertiliser)
|
|
minimum
|
maximum
|
average
|
|
Anhydrous ammonia
|
0
|
3.6
|
1.8
|
|
Urea
|
0
|
3.6
|
1.8
|
|
Ammonium nitrate
|
0
|
3.6
|
1.8
|
|
Ammonium sulphate
|
3.6
|
7.2
|
5.4
|
|
DAP (18:20)
|
1.8
|
5.4
|
3.6
|
|
MAP (10:22)
|
3.6
|
7.2
|
5.4
|
|
Goldphos (0:18:0:10)
|
|
3
|
|
|
Superphosphate
|
|
|
nil
|
|
Muriate of potash
|
|
|
nil
|
Removal of plant products
The removal of plants products leaves the soil more
acidic. Highest acidification rates occur when hay is cut and fed on a
different paddock or sold off the property. Similarly, the use of night
paddocks transfers alkalinity from the other paddocks to the night
paddocks.
Figure 3:
Lime required to counteract acidity caused
by product removal.
|
Plant product
|
Lime requirement*
|
|
Lucerne hay
|
60
|
|
20% subclover/annual grass
|
15
|
|
40% subclover/annual grass
|
30
|
|
60% subclover/annual grass
|
40
|
|
80% submedic/annual grass
|
50
|
|
perennial ryegrass hay
|
40
|
|
cereal hay
|
22
|
|
phalaris/cocksfoot hay
|
30
|
|
wheat grain
|
5 - 10
|
|
barley grain
|
5 - 10
|
|
Wool
|
14
|
|
Milk (1000L)
|
4
|
*kg CaCO3 per tonne
Soil pH
Soil pH is a measure of the acidity or alkalinity of a
soil. Acid soils have a pH lower than 7, alkaline soils have a pH greater
than 7. The lower the pH, the more acid the soil.
There are two common ways of measuring soil pH - in water
and in calcium chloride. The latter is preferred for acidic sols because
results are generally more consistent. pH measured in calcium chloride are
generally 0.5 to 1 pH unit lower than if it is measured in water. Make
sure you know which method has been used!
|
Animal product removal
The removal of animal products also cause soil
acidification but rates are generally low.
For example at normal
management levels for milk, wool or meat production, the acidity produced
is only equivalent to about 1 to 10 kg of lime per ha per year.
Note: Superphosphate does not directly cause acidity,
but may increase the process indirectly by promoting better pasture growth
or higher productivity.
Symptoms of Soil Acidity
The following symptoms tend to indicate a soil acidity
problem.
-
Reduced yields
-
Poor plant vigour
-
Uneven pasture and crop growth (especially acid
sensitive plants).
-
Poor establishment and persistence of pasture species
such as lucerne and phalaris where previously they grew well.
-
Poor nodulation of legumes.
-
Stunted root growth.
-
Persistence of acid-tolerant weeds (eg sorrel and
geranium).
-
Increased incidence diseases
-
Abnormal leaf colours
-
A soil pH test is needed to confirm a soil acidity
problem.
What does acidity do?
Soil acidity has a negative impact on fertility,
biological activity and plant productivity.
Plant tolerance and productivity
Species and varieties with low tolerance to acidity will
decline in productivity and persistence. Greater reliance is placed on
acid tolerant plants that are generally not as productive.
Soil fertility
Soil pH influences nutrient availability. In strongly acid
soils, potassium, calcium and magnesium are depleted due to leaching. Low
levels of calcium and magnesium can also cause stock health problems such
as milk fever and grass tetany. A lack of calcium can cause soil
structural problems.
Aluminium, if present in the soil, becomes available once
pH(CaCl2) goes down to less than 5. Aluminium is toxic to plants and
severely restricts root growth. Acids attack soil minerals and increase
net loss of nutrients from the soil eg. Mn, Cu, Zn.
In some soils Manganese toxicity will develop around pH
(CaCl2) 5.0 although this is unusual in most high rainfall SA soils
Figure 4:
Effects of acidic
soil on plant nutrients.
|
Nutrient
|
Action
|
Outcome
|
|
Potassium
|
Depleted
due to leaching
|
Stock
health problems
|
|
Calcium
|
Depleted
due to leaching
|
Poor
soil structure
|
|
Magnesium
|
Depleted
due to leaching
|
Poor
soil structure
|
|
Phosphorus
Molybdenum
|
Deficiency
due to fixation
|
Poor
pasture growth
|
|
Aluminium
|
Excess
|
Toxic
to plants if soil reserves are high
|
|
Iron
|
Excess
|
Ties
up other nutrients eg P
|
Biological activity
Soil acidity reduces and even stops the activity and
survival of useful soil organisms such as:
-
nitrogen fixers
-
decomposers
-
nutrient recyclers
Organic mats often form on the soil surface as a result of
reduced biological activity and organic matter not being broken down.
Soil Structure/Clay Degradation
The leaching of nutrients and increased availability of
clay minerals such as Al and Fe can result in a decline in soil structure
and some irreversible damage to the clay content of soil.
Off-site effects
As a result of poor pastures, limited growth and shallow
root depth, recharge under acid soils is greater than under productive
perennial pastures. This will contribute to rising water tables and an
increase in the salinity of streams and dryland salinity.
Streams are also more likely to contain nutrients leached
out of the soil due to the acidic conditions.
Benefits of liming
Raises soil pH.
A well balanced soil pH is important for: soil fertility
and nutrient availability plant species that can be grown biological
activity of the soil
Pasture vigour and productivity
Lime application increases pasture productivity. Trials
throughout the Mt Lofty Ranges showed increases in productivity up to 35%.
Livestock health
Increased calcium and magnesium levels in the plant helps
to overcome problems such as grass tetany in cattle.
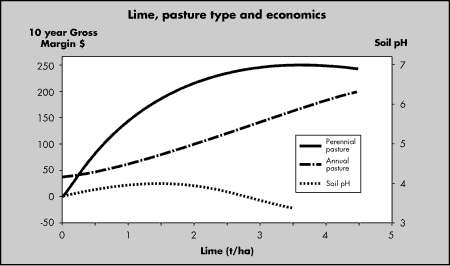
Economics
Research data shows that responses to lime can be
profitable (Fig 1 and 2). Most economic advantage is achieved by liming
highly productive or perennial pastures.
Perennial pasture: phalaris/subclover. Most
economic rate is 3.5t/ha, which is sufficient to increase subsoil
pH.
Annual pasture: annual ryegrass/subclover. Most
economic rate is 1.5t/ha, but is insufficient to improve subsoil acidity.
Figure 5:
Economics of applying lime to
perennial or annual pastures (Data from NSW Agriculture, Wagga
Wagga)
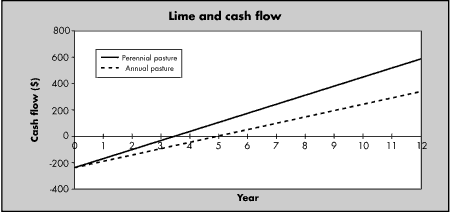
Figure 6:
Net cash flow after applying lime
to perennial or annual pastures. Lime applied at 3.7t/ha at total cost of
$235/ha. (Data from NSW Agriculture, Wagga Wagga)
Management of acidity
|
Prevention is better than cure!
As acidity is a slow
process and the correction of acidity by liming is also slow where
possible soils need to be limed before acidity is having an effect |
What can we do?
Do not assume that every paddock has the same soil pH
or acidification rate!
Management options
-
Apply liming material at a rate based on pH, soil
type, land use (see next section).
-
Use acid-tolerant plants eg cocksfoot, some clovers.
This is a short term option only as the soil continues to acidify with
associated consequences.
-
Reduce the rate of acidification to a minimum.
-
Sow perennial pasture - deep rooted, more summer
active, reduce N leaching.
-
Use fertilisers wisely - match plant requirements,
monitor plant and soil levels, and use least acidifying N
fertilisers.
-
Feed hay onto paddock in which it was cut where
possible - recycles nutrients and alkalinity.
-
Rotate grazing paddocks.
-
Irrigation with bore water in the Mt Lofty Ranges
and SE often applies carbonate which helps to neutralise
acidity.
-
Buy in hay/grain where practical.

3.2 Soil Acidity and Liming
[ Back ] [ Next ]
|
