
5.4 Key Nutrients
Soil analyses for phosphorus, potassium, surface pH
and organic carbon are best done on a bulked sample from approximately 30
sub-samples from the 0-10cm surface layers, collected from the same soil
type. With increasing interest in sub-soil fertility, judicious sampling
of deeper layers is recommended, especially for sulphur and nitrate.
Nitrogen
Nitrogen deficiency is probably the most widespread
nutrition problem in South Australian agriculture. Soil nitrogen (N) can
take two basic forms in soils: organic and inorganic. Organic forms
include that associated with soil organic matter, plant residues, soil
organisms and animal waste; at least 90% of soil nitrogen originates from
organic sources. Inorganic N can take the form of ammonium, nitrite and
nitrate, but is typically dominated by nitrate in agricultural soils. The
conversion of organic to inorganic N is called mineralisation and is
driven by micro-organisms.
The micro-organisms use the organic N as a nutrient
source. Any organic N in excess of their maintenance or growth
requirements is released to the soil as ammonium. This ammonium is
converted by specific bacteria to nitrate that can then be used by plants.
The conversion of ammonium to nitrate N is called nitrification.
The nitrogen cycle illustrated in Figure 27 demonstrates the flow of plant residue
N, through soil micro-organisms and soil organic matter and into the
inorganic fraction as ammonium.
Organic carbon percentage (OC%) gives an indication
of likely plant available N. The higher the OC% the more N available, but
seasonal conditions will control the mineralisation of this N.
Nitrate levels vary considerably and should be
measured in the whole root zone and deep nitrate testing at 60cm is
becoming more widely used. SAP nitrate testing and near infra-red
reflectance (NIR) are used to assess the nitrogen status of growing crops.
Rhizobia, nitrogen-fixing bacteria, form a symbolic
relationship with leguminous plants, eg clover, lupins. The Rhizobia
provide N to the plant and in return the plant supplies sugars to the
bacteria. This is an important source of crop N.
|
Figure 2:
Fate of fertiliser N applied to a crop on a clay loam
soil
|
|
Fate of Fertiliser |
% of applied |
|
Taken up by plant -
into above ground parts
|
40 -
60
|
|
Incorporated in the
soil's organic matter
|
20 -
50
|
|
Mineral form in the
soil (clay - ammonia complex)
|
5 -
20
|
|
Lost by
denitrification and volatilisation
|
2 -
30
|
|
Lost by leaching
|
2 -
10
|
|
Source Hi-Fert, Plant Nutrition and Soil Fertility, 1997
|
When assessing a crop N requirement, the following
factors should be considered:
Paddock
history
Crop
targets
Phosphorus
|
Figure 3:
Phosphorus cycling in a wheat medic rotation.
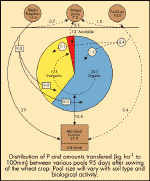
Click for large image |
Phosphorus is almost universally deficient in
Australian soils in their natural state. Added phosphorus becomes attached
to clay particles, organic matter and compounds of calcium, iron and
aluminium. Only part of the total content is available to plants. The
source of phosphorus will determine how much is readily available to
plants at a given point in time. This is illustrated in Figure
3.
Soils with a high pH and those with high levels of
iron, calcium and aluminium have a reduced capacity to supply phosphorus,
because in these situations, phosphorus is less soluble. These soils,
together with sandy soils in high rainfall areas, which are prone to
leaching, are the most likely to be phosphorus deficient.
Potassium
Except on very sandy soils in the higher rainfall
districts, deficiency of potassium in South Australia’s agricultural
soils is rare.
This is because the rocks and sediments from which
most soils are formed contain clay minerals naturally high in potassium.
Deficiencies are most likely where large amounts of potassium have been
removed in farm products, particularly hay.
Trace Elements
Trace elements are required by plants and are often
only present in small quantities. Deficiency symptoms may only occur
sporadically. These may be due to seasonal conditions, reduced
availability caused by addition of other fertilisers, or a whole lot of other factors
|
Figure 4:
Extractable Phosphorus (Colwell) (mg/kg)
|
|
|
Non- calca-
reous Crops |
Calca-
reous crops |
Pastures |
Potatoes |
Other Veg' |
Vines/
apples |
|
Very Low |
< 10 |
< 15 |
< 10 |
< 20 |
< 40 |
|
|
Low |
10 - 20 |
15 - 25 |
10 - 18 |
20 - 40 |
40 - 80 |
|
|
Marginal |
20 - 30 |
25 - 35 |
18 - 25 |
40 - 55 |
80 - 12 |
|
|
Adequate |
30 - 45 |
35 - 45 |
25 - 45 |
55 - 100 |
120 - 150 |
> 80 |
|
High |
> 45 |
> 45 |
> 45 |
> 100 |
> 150 |
|
Source: Primary
Industries and Resources S.A.
|
|
Figure 5:
Extractable Potassium (mg/kg)
|
|
|
Permanent pastures |
Potatoes |
Other vegetables |
|
Low |
< 80 |
< 120 |
< 150 |
|
Marginal |
80 - 120 |
120 - 250 |
150 - 250 |
|
Adequate |
120 - 250 |
> 250 |
> 250 |
|
High |
> 250 |
|
|
Source: Primary
Industries and Resources SA
|
The situations where the main trace element
deficiencies are most likely to occur are:
Copper: Acid and calcareous sands
Manganese: Highly calcareous soils
Zinc: All soils, especially calcareous and sandy types
Molybdenum: Sandy, acid soils in high rainfall areas
Iron: Calcareous soils
Boron: Leached sandy soils
Note that boron can also be toxic; if test
results are more than 15ppm
Soil tests for manganese and iron currently have
little interpretative value, but values of less than 0.2ppm for copper and
0.5ppm for zinc indicate likely deficiencies.
Plant tissue testing is necessary to establish if
plants are obtaining adequate trace elements from the soil.
Sulphur
Sulphur occurs in both organic matter and as sulphate
compounds in the soil. Sulphur deficiencies are not widespread and are
most likely in sandy soils in high rainfall areas and in soils low in
organic matter. Removal of sulphur in produce reduces soil sulphur levels.
Additions are now less, as many fertilisers are now low in sulphur.
Calcium
Calcium occurs in limestone, calcrete and soft
‘lime,’ all forms of calcium carbonate and a common constituent of
South Australian soils. It also occurs in gypsum (calcium sulphate) and
attached to clay minerals. Calcareous soils and soils with high clay
contents, are unlikely to suffer calcium deficiencies.
Magnesium
Like potassium and calcium, magnesium is present in
large amounts attached to the clay minerals of most soils. Leached sandy
soils are the most prone to deficiencies.

5.4 Key Nutrients
[ Back ] [ Next ]
|
