
5.3 Soil pH
PH is a measure of the hydrogen ion concentration
acidity or alkalinity of the soil. Measured on a logarithmic scale, a soil
at pH5 is 10 times more acidic than a soil at pH6 and 100 times more
acidic than a soil at pH7. A neutral soil has a pH of 7. A soil is acidic
if the pH is less than 7 and alkaline if the pH is greater than 7. pH can
affect the availability of plant nutrients and toxins and the activity of
many essential micro-organisms. The pH of a soil may influence the choice
of crops grown and the type of soil organisms that are present and
function in the soil.
The majority of agricultural species prefer
approximately neutral pH levels. Soils that are excessively acid or
excessively alkaline cause reduced productivity.
|
Figure 1:
The pH scale and the pH of some common substances.
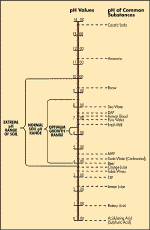
Source: Hi-Fert, Plant Nutrition and Soil Fertility, 1997
|
Acidity
Soils are considered acidic if the pH measured in
water is less than 7 and strongly acid if the pH is less than 5 (both
measured in water).
Some soils, particularly those with low clay content
in high rainfall areas, are naturally acidic. Induced acidification,
caused by accelerated accumulation of acids under certain land management
practices, can be a significant problem. Areas most affected are the
higher rainfall districts such as the Mount Lofty Ranges, South East,
Kangaroo Island and Lower Eyre Peninsula.
Acidification is caused by:
-
Accumulation of organic matter, which produces
organic acids as it decomposes. Increasing the organic matter of a soil
provides many benefits and liming can be used to maintain good soil pH.
-
Addition of nitrogen to the soil via ammonium
fertilisers or fixation of atmospheric nitrogen by leguminous plants, both
result in the production of nitrates, which can hydrolyse to nitric and
nitrous acid.
-
Removal of alkaline elements (cations) in crops. Figure
24 illustrates the equivalent weight of lime required to replace
the alkaline elements removed by a range of crops.
-
Therefore, intensive cropping will result in a fall
in soil pH over time. In alkaline soils this could have positive effects
on production, but if soil is lower than pH6, crop performance can be
reduced.
The susceptibility of a given soil to acidification
is determined by its ‘buffering capacity,’ or ability to resist pH
change. Soils with high clay content, high cation exchange capacity and
organic matter levels generally have higher buffering capacities.
Alkalinity
Alkalinity is usually an inherent characteristic of
soils, although irrigation can increase the alkalinity of saline soils.
Soils made alkaline by calcium carbonate alone rarely have pH values above
8.5 and are termed ‘calcareous’.
|
Figure 2:
The
equivalent weight of lime (CaCO3) required to replace the alkalinity
exported by crops
|
|
Per 1t of produce
removed
|
Lime requirement to
replace alkaline elements (kg)
|
|
Lucerne
hay
|
70
|
|
Clover
hay
|
40
|
|
Grass hay
|
25
|
|
Maize
silage
|
40
|
|
Wheat
grain
|
9
|
|
Lupin
grain
|
20
|
|
Wool
|
14
|
|
Milk
(1000 l)
|
4
|
Source: Slattery,
Ridley - Department of Agriculture Victoria
|
Alkaline soils with pH values higher than 8.5 usually
have significant exchangeable sodium (sodic soils).
Consequences of Soil Acidity and Alkalinity
Many of the adverse consequences of excessively high
or low soil pH values are related to plant nutrient availability.
As Figure
25 indicates, the availability of individual elements varies withy
pH. For example, availability of aluminium increases in acid conditions.
This can lead to toxicity.
Plants vary in their ability to extract nutrients
from the soil and, for this reason, some plants are able to perform better
in acid soils, while others prefer a higher pH (see Figure
4).
There are also some specific consequences of acidity
and alkalinity.
In acid soils:
The rhizobia bacteria responsible for nitrogen
fixation have specific pH requirements and most are less active at low pH,
except for strains with lupins and sub-clovers. Reduced vigour of many
leguminous plants is a major consequence of soil acidification.
|
Figure 3:
The effect of pH (based on
hydroponic solutions) on the availability of plant nutrients
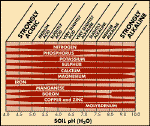
Click for larger image
|
Aluminium and manganese become so readily available
at low pH (approx <pH5 (H2O)) that toxicity may occur. Stunted growth and leaf necrosis, respectively, can result.
Extractable aluminium levels of less than 2ppm affect very
sensitive species, such as lucerne, while tolerant species, such as oats,
will withstand values up to 13ppm.
In alkaline soils:
Root growth is inhibited in strongly alkaline soils,
possibly due to the plant's inability to absorb nutrient elements, such as
zinc, which cannot move through the soil and must be present at the
growing root tip.
Soil pH is an
important factor when considering what species to grow where.
|
Figure 4:
The
optimum pH range for different crop, pasture and horticultural species
|
|
Preferred
Soil Reaction: Field Crops
|
|
acid soil |
acid to neutral soil |
neutral to alkaline soil |
|
Cotton
Cowpeas
Lupins
Linseed
Miller
Oats
Peanuts
Pyrethrum
Rice
Triticale
Soybean
Sugar cane
Sunflower
Vetch
Wheat
|
Barley
Canola
Corn
Faba beans
Kale
Kohlrabi
Mung beans
Navy bean
Rye
Sorghum
|
Barley
Canola
Chickpeas
Field peas
Lentils
Safflower
Vetch
Wheat
|
| |
|
Preferred
Soil Reaction: Vegetable Crops
|
|
acid soil |
acid to neutral soil |
neutral to alkaline soil |
|
Blackberry
Blueberry
Endive
Fennel
Pineapple
Potato
Pumpkin
Rhubarb
Shallot
Strawberry
Sweetcorn
Sweet potato
Watermelon
Raspberry
Squash
Tomato
Turnip
|
Beans
Brussell sprout
Carrot
Collard
Cucumber
Eggplant
Garlic
Gherkin
Mustard
Parsley
Peas
Pepper
Radish
Parsnip
Salsify
Spinach
Watercress
|
Asparagus
Beetroot
Broccoli
Cabbage
Cauliflower
Celery
Cress
Leek
Lettuce
Muskmelon
NZ spinach
Okra
Onion
|
| |
| Preferred
Soil Reaction: Fruit Crops |
|
acid soil |
acid to neutral soil |
neutral to alkaline soil |
|
Apple
Macadamia
Nectarine
|
Apricot
Citrus
Grape
Peach
Pear
|
Almond
Cherry
Pistachio
Plum
|
| |
|
Preferred
Soil Reaction: Cut Flowers
|
|
acid soil |
acid to neutral soil |
neutral to alkaline soil |
|
Azalea
Protea
Hydrangea
|
Rose
Waratah
Tulip
|
Carnation
Daffodil
|
Source: Hi-Fert,
Plant Nutrition and Soil Fertility, 1997
|
Measuring soil pH
Soil pH should be monitored regularly in the
surface and subsurface, as small changes can result in changes in nutrient
availability.
Lime should be applied to cropping soils where
acidity is <pH5.8 (H2O).
In the field, pH can be tested using either a
pocket pH meter or a field test kit. Using a meter requires that the soil
be in a suspension of water pH (H2O) or calcium chloride pH (Cau2), whilst
the test kit uses a powder sprinkled on the soil sample. Indicator strips
are also available, but a recent study by CSIRO Land & Water comparing
field tests for measuring pH found these to be less accurate, especially
for high pH soils.
If your field results suggest pH is near a
critical level, samples should be tested in the laboratory.
When a pocket pH meter is used, it is crucial to
calibrate this correctly using a standard buffer solution. This can be
ordered from the supplier of the meter.

5.3 Soil pH
[ Back ] [ Next ]
|
